On December 6, 2024, Chongqing Precision Biotech’s self-developed MC-1-50 cell preparation obtained clinical trials approval from the Center for Drug Evaluation (CDE) of the NMPA to conduct clinical trial for the treatment of refractory systemic lupus erythematosus in patients aged 18 and above. So far, Precision Biotech has obtained the implied license of 4 CAR-T cells Class I new biological drug, covering 10 indications including hematological tumors, solid tumors, and autoimmune diseases.
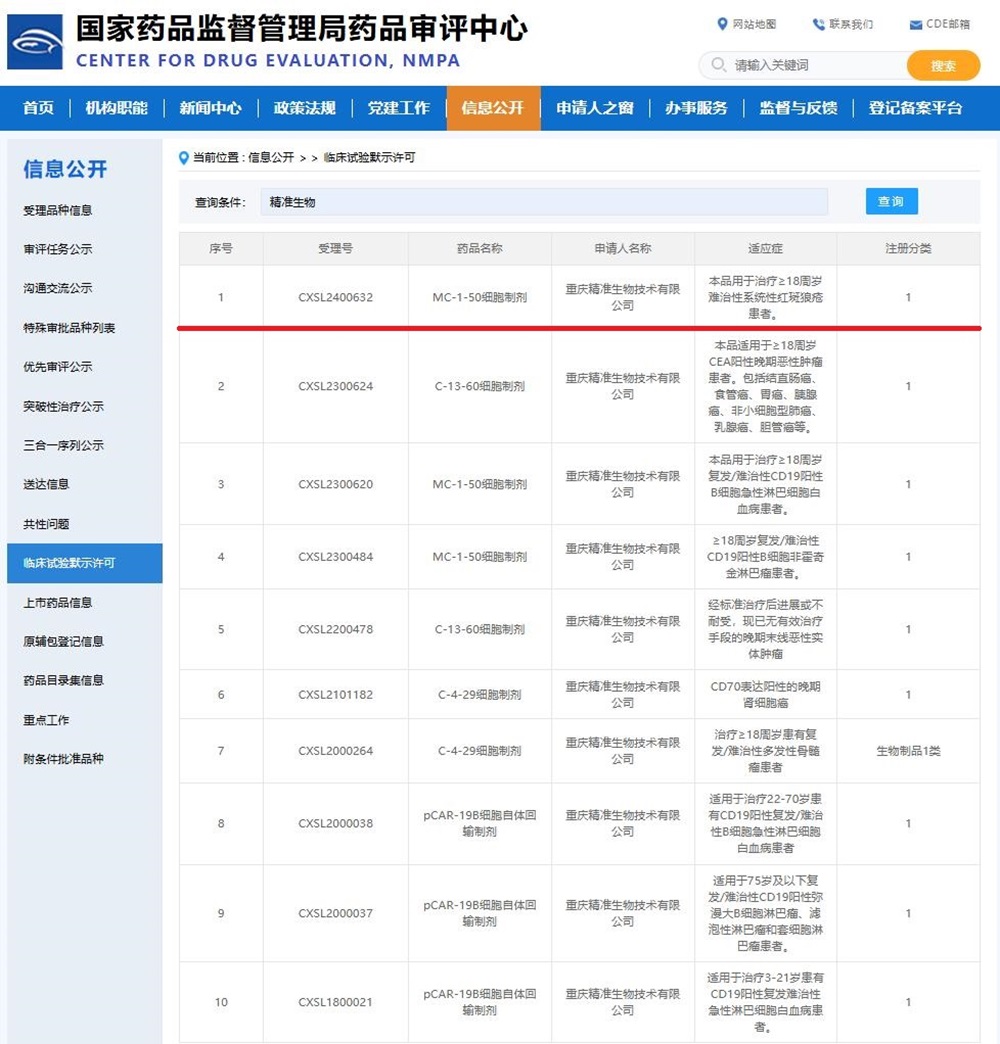
MC-1-50 is a second-generation CAR-T cell therapy targeting CD19, which is independently developed by the company. By using a new, faster and more effective preparation scheme of CAR-T cells, it can realize a lower dose, less side effects and more lasting CAR-T product. This new indication for the MC-1-50 cell preparation marks an extension of its application beyond B-cell acute lymphoblastic leukemia and non-Hodgkin’s lymphoma. It also expands the company’s product range into the autoimmune disease sector, offering a novel treatment option for patients suffering from refractory systemic lupus erythematosus (SLE).
At the groundbreaking ceremony, Jia Hui, a member of the Party Working Committee of the Chongqing High-Tech Zone in the Western Science City and Deputy Director of the MSLE is a chronic, multi-organ system involved autoimmune disease, known as the “undying cancer,” affecting the health and quality of life of millions of Chinese people. Traditional treatments mainly focus on controlling disease activity, improving clinical symptoms, preventing and reducing relapses. Less than 1% of patients are able to achieve complete remission without medication, often accompanied by severe side effects. In recent years, CAR-T cell therapy has shown remarkable efficacy in the field of autoimmune diseases. This innovative therapeutic approach is set to play a pivotal role in managing SLE, offering more effective and enduring treatment options for patients. It promises to substantially enhance their quality of life and solve the current therapeutic challenges they face.
This product’s Phase I clinical study data was first disclosed by Professor Jianhua Mao’s team from the Affiliated Children’s Hospital of Zhejiang University School of Medicine at the American Society of Nephrology Kidney Week (ASN Kidney Week) in 2024 in the form of an oral presentation. 11 SLE patients treated with MC-1-50 showed significant reductions in SLEDAI-2K scores after infusion. As of the data cutoff date, they remained in a drug-free remission state. Adverse reactions were all mild and improved rapidly after symptomatic treatment.
Autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, and antiphospholipid antibody syndrome, as the second-largest disease field globally after cancer, have always held tremendous opportunities. In 2023, three of the top ten highest-grossing pharmaceuticals worldwide were for treating autoimmune diseases. According to Frost & Sullivan’s forecast, by 2030, the global market for autoimmune disease medications could expand to $176 billion.The market for autoimmune disease drugs in China is expected to reach $25 billion, becoming an important incremental source for the global autoimmune disease drug market. The successful application of CAR-T therapy in autoimmune diseases has paved the way for broader applications of this groundbreaking technology and products in various fields.




