From May 31-June 4, 2024 the American Society of Clinical Oncology (ASCO) was held in Chicago, USA. At the oral report session of Gastrointestinal Cancer - Colorectal and Anal, held on June 3rd local time, Professor Fang Weijia's team from the First Affiliated Hospital of Zhejiang University reported the latest clinical trial research results of a new CEA-targeted CAR-T cell preparation jointly conducted with Chongqing Precision Biotech for the treatment of CEA-positive advanced/metastatic solid tumors. This study used intraperitoneal injection, providing new strategies and ideas for the treatment of solid tumors.

Abstract #3514
Phase I trial of hypoxia-responsive CEA CAR-T cell therapy in patients with heavily pretreated solid tumor via intraperitoneal or intravenous transfusion.
Background:Chimeric antigen receptor (CAR) T cell therapy has been successful in hematologic malignancies but not in solid tumors. Homing capacity and tumor microenvironment are key issues to limit CAR-T efficacy to solid tumors. Our previous study has shown that hypoxia-responsive CAR-T cells exhibit reduced exhaustion and enhanced efficacy in solid tumors (Cancer Res 2024). Here, we aimed to optimize the administration route by comparing safety and efficacy of our newly established CEA CAR-T through intraperitoneal (I.P.) and intravenous (I.V.) infusions in solid tumor patients who failed to standard therapy.
Methods:In this phase I, open-label, dose-escalation clinical trial, eligible patients were CEA positive and relapsed or refractory to at least second-line treatment. CAR-T cells was administered via I.P. or I.V. with dose escalation including low dose of 1-3 x106 CAR+/kg and high dose of 4-6 x106 CAR+/kg. Adverse events were graded by CTCAE 5.0. Tumor responses were evaluated using RECIST 1.1.
Results:Between June 2022 to Jan 2024,40 pts were enrolled and infused CAR-T cells, including 35 CRC pts, 3 GC pts, 1 NSCLC pts and 1 BTC pts. There were 16 pts in the I.P. group and 24 pts in the I.V. group. CRS was observed in 62.5% of the pts at grade 1-2, and no ICANS or treatment related death was reported. Grade 1-2 mucositis was reported in 25% of the patients. Immune-mediated diarrhea and colitis of all grades occurred in 32.5% of the patients, including 17.5% grade 3 events. And 100% of patients experienced grade 3-4 hematologic toxicity. In terms of efficacy, the I.P. group displayed a higher objective response rate (ORR) of 25% (4/16), compared to 8% (2/24) in the I.V. group, alongside a superior disease control rate (DCR) of 88% (14/16) in I.P. compared to 67% (16/24) in I.V. group. Notably, within the high-dose group receiving I.P. infusion, the ORR increased to 28.5%. Moreover, the responded patients in the high-dose group all had sustained tumor remission for more than 5 months, including one patient who had a 76% target lesions regression at six months follow up. CAR-T cells demonstrated robust expansion in both the I.P. and I.V. groups, while no significant difference was found in maximum concentration.
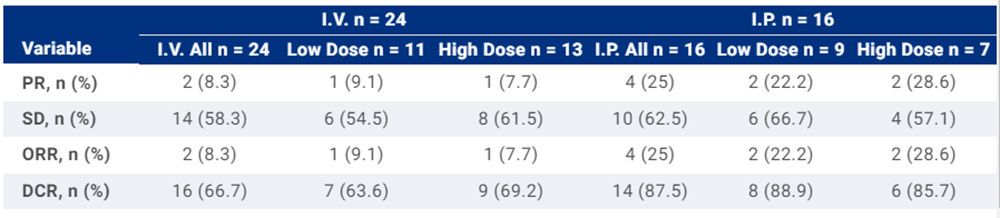
Conclusions:The CEA CAR-T cell therapy had acceptable toxicity via I.P. or I.V. infusion. We have observed a promising antitumor potential by I.P. infusion and a prolonged tumor remission in high-dose group. Further investigation is needed to highlight these findings and the corresponding mechanisms. Clinical trial information: NCT05396300.
link:https://ascopubs.org/doi/10.1200/JCO.2024.42.16_suppl.3514
CEA Technology R&D Platform of Chongqing Precision Biotech
Carcinoembryonic antigen (CEA) is a glycoprotein that is normally expressed during the development of carcinomatous embryos and also in normal colonic mucosa. CEA is overexpressed on the surface of many cancer cells, including colorectal cancer, 70% of non-small cell lung cancers, and approximately 50% of breast cancers, and is rarely expressed on normal cells except for low expression on gastrointestinal epithelial cells.
Because of its high heterogeneity and complexity of tumor microenvironment, solid tumor has been the focus and difficulty of clinical treatment. The new technology of resisting and/or reversing tumor immunosuppressive microenvironment is a common problem urgently to be solved in the field of current tumor immunotherapy. Chongqing Precision Biotech has conducted in-depth research on CAR-T cell therapy for solid tumors, and developed PhiCAR ® and RESCAR ® tumor microenvironment resistance technology platforms for solid tumor microenvironment. This CAR-T product targeting CEA is developed based on the above two platforms.
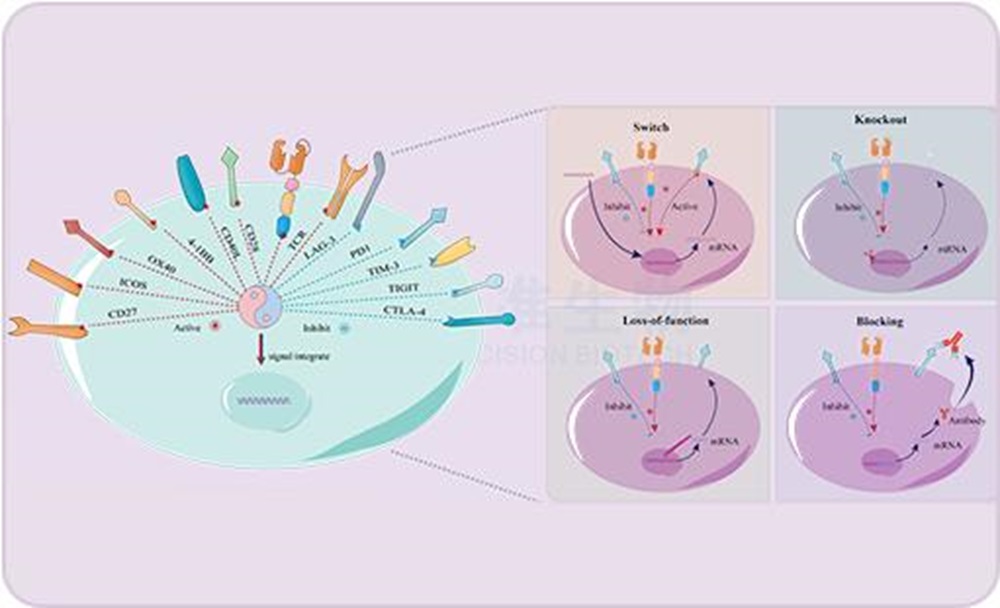
RESCAR ® Platform: Say "no" to inhibitory signals, which break down the tumor suppressive microenvironment and stimulate the active potential of immune cells.
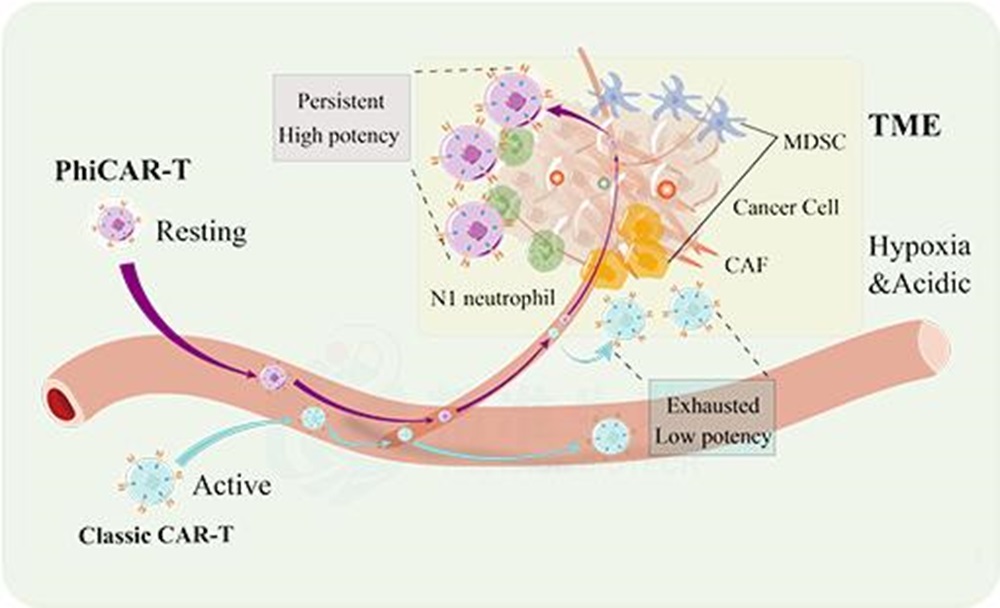
PhiCAR ® platform: to break the barrier of tumor physical microenvironment, improve the survival of immune cells in tumor microenvironment, promote the local accumulation of immune cells in tumor, and reduce the depletion of non-"working state" immune cells.
Chongqing Precision Biotech's cell therapy products for solid tumor are fully adopt the above-mentioned platforms. In addition to the CEA target products reported in the oral session this time, Chongqing Precision Biotech also selected PSCA CAR-T products for online publication at this ASCO conference.
Abstract #e14574
A novel co-receptor with mutated TIGIT to enhance PSCA CAR-T therapy for bladder cancer.
Background:TIGIT plays a crucial role in immune regulation, particularly in tumor environments, by acting as an inhibitory receptor. Leveraging its interaction with ligands like CD155 on tumor cells, we developed an innovative CAR-T cell therapy using a mutated TIGIT co-receptor to overcome inhibitory effect induced by CD155, together with targeting Prostate Stem Cell Antigen (PSCA) CAR, chosen for its high expression in bladder and other cancers versus its minimal presence in normal tissues.
Methods:We initiated our approach by inducing strategic mutations in TIGIT, selecting a variant with heightened affinity for CD155. Bio-Layer Interferometry (BLI) analysis confirmed the variant's high binding affinity (KD = 1.635 nM). This mutated TIGIT, combined with CD28's signaling components, was integrated as a co-receptor in our CAR construct. For PSCA targeting, we isolated a high-affinity ScFv against PSCA from a fully human phage display library. The specificity of this ScFv for PSCA was rigorously validated using Cell Membrane Protein Targeting Catcher (CMPTC) technology, covering over 6000 human cell membrane proteins.
Results:In our comparative analyses, CAR-T cells featuring a mutated TIGIT co-receptor (T-PSCA-CAR-T) matched the cytotoxicity of traditional second-generation BBz-structured CAR-T cells (PSCA-CAR-T) against PSCA-expressing HT-1376 bladder cancer cells. However, T-PSCA-CAR-T cells distinguished themselves by producing significantly higher IFN-γ levels (3805.683±65.506 vs 1271.712±39.768 pg/mL). Our in vivo tumor model, utilizing luciferase-expressing HT-1376 cells, demonstrated T-PSCA-CAR-T's enhanced anti-tumor efficacy, as evidenced by a more significant reduction in bioluminescence signal intensity (average decreasing ratio: 0.02 for T-PSCA-CAR-T vs 0.35 for PSCA-CAR-T; p < 0.05). These results underscore the efficacy of the mutated TIGIT co-receptor in improving CAR-T cell therapy.
Conclusions:Our study signifies a transformative advancement in CAR-T cell therapy by incorporating a mutated TIGIT co-receptor, markedly improving therapeutic outcomes in bladder cancer treatment. Utilizing PSCA as a model target not only validates the efficacy of this novel approach but also paves the way for its application in a broader range of cancers, representing a significant leap in the field of immunotherapy.
link:https://ascopubs.org/doi/10.1200/JCO.2024.42.16_suppl.e14574
About Precision Biotech
Chongqing Precision Biotech Co., Ltd. was established in 2016 as a high-tech enterprise jointly established by a technical team with Professor Qian Cheng as the leader, a well-known scientist of precision medicine, and a listed company, Zhifei Bio (Zhirui Investment). The company takes gene editing and synthetic biology technology as the breakthrough point, mainly engaged in the development and application of gene and cell medicine, and has become the leading enterprise in the field of CAR-T/NK in China. The company is determined to become a global, innovative and pioneering biopharmaceutical enterprise.

As a scientific and technological innovation enterprise, the company grasps the key technologies in the field of cells, has applied for more than 150 patents, and owned over 50 authorized patents for invention, and also laid out patents in developed countries and regions. We has implemented and promoted more than 10 CAR-T R&D pipelines for leukemia, lymphoma, myeloma, gastrointestinal cancer, lung cancer, liver cancer, breast cancer and pancreatic cancer, and obtained implied licenses for four Class I CAR-T cell biological new drugs and nine indications. The first domestic self-developed pCAR-19B cell autologous infusion preparation for the treatment of childhood leukemia has been approved as a "Breakthrough Therapy Designation". Meanwhile, we has achieved technical breakthroughs in the treatment of renal cancer, colorectal cancer, gastric cancer and other solid tumors with CAR-T, and relevant products have been approved for clinical registration and are actively promoting clinical drug registration. We will continue to adhere to our mission of "innovation for health", make innovations for cell therapy technology and focus on malignant tumor treatment. Based on the domestic and international dual-cycle strategy, we will implement the strategy of globalization of technical resources and internationalization of marketing, build a regional cell preparation base in China, explore the establishment of overseas R&D branches, and strive to build a biomedical enterprise with global competitiveness.
Disclaimer: This article is only for the exchange of industry information. The content of this article only represents the author's point of view, not the position of Precision Biotech, nor does it mean that Precision Biotech supports or opposes the views in this article. This article does not constitute any value judgment, investment suggestion or treatment recommendation scheme. If necessary, please consult a professional or go to a regular hospital.
Copyright Note: This article is a team's integration of information. Individuals are welcome to share and forward it. Media or institutions are not allowed to reprint it to other platforms in any form without authorization (if you need to reprint it, please leave a message in WeChat official account backstage or in the message area below for authorization).








