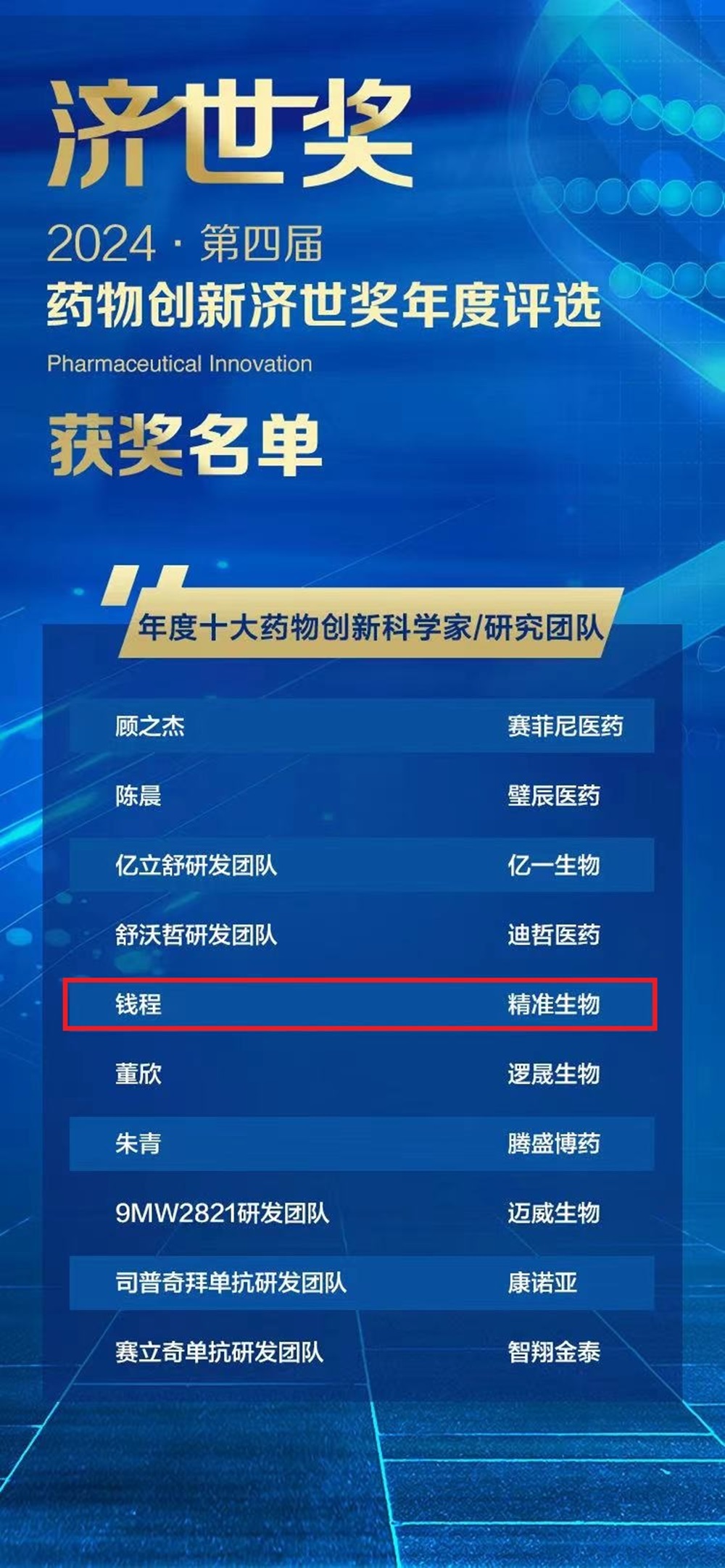
On June 20th, the high-profile 4th Annual Selection and Awarding Conference of Drug Innovation Award was held in Shanghai. Professor Qian Cheng, Chairman and Chief Scientist of Chongqing Precision Biotech, was awarded the title of "Top Ten Drug Innovation Scientists in 2023".
About Professor Cheng Qian- Chief Scientist
Professor Cheng Qian is the Director of National International Joint Research Center for Precision Medical Biotherapy of Ministry of Science and Technology of China and Chief Scientist of the Key Research and Development Program of the Ministry of Science and Technology of China. He is also specially-appointed expert of the "TOP 100 People Program" in Chongqing, and the honorary winner of the Top Ten Chongqing Scientific and Technological Innovators of the year.
Professor Qian Cheng, as a well-known international expert in the field of precision medicine immunotherapy, has devoted himself to his research in the field for 30 years, published more than 200 papers in the international authoritative SCI journals, with a total impact factor of 1500. He was cited by SCI journals for more than 20,000 and has been selected into the list of "Elsevier Highly Cited Chinese Researchers" for many times, and applied for more than 80 PCT and national invention patents. At present, more than 2000 cases of terminal cancer patients have been successfully treated and cured by his immune cell technology throughout the country.
Professor Qian Cheng led his R&D team to create Chongqing Precision Biotech with the support of Zhifei Bio (Zhirui Investment). Taking gene editing and synthetic biology technology as the breakthrough point, the company is mainly engaged in the development of gene and cell drugs, implemented and promoted the research and development of more than 10 CAR-T pipelines, applied for more than 150 patents at home and abroad (including subsidiaries), and obtained the implied license of 4 CAR-T cells Class I new biological drug and 9 indications.
Among them, China's first CAR-T cell product for childhood leukemia developed by ourselves has been approved as "Breakthrough Therapy Designation" . Besides, we developed the world's first dual-target CAR-T registered clinical product targeting both CD70 and BCMA, and developed the world's first CEA CAR-T product approved to carry out registered clinical trials at the national level. The high-safety short-term CAR-T manufacturing technology developed by us has the advantages of short preparation period, low cost, good curative effect and less side effects, which makes it more accessible for patients to receive CAR-T for cancer treatment through outpatient service. Once our related products are on the market, it will fill the gaps in precision medicine fields in China, continuously expand the domestic biomedical industry, so that people can use and afford innovative drugs as soon as possible!




