Recently, Chongqing Precision Biotech obtained the seventh IND acceptance for CAR-T products. This is the first IND officially applied for MC-1-50,which developed by the company's PRIMCAR platform. MC-1-50 is a second-generation CAR-T cell therapy targeting CD19, which is independently developed by the company. By using a new, faster and more effective preparation scheme of CAR-T cells, it can realize a lower dose, less side effects and more lasting CAR-T product.
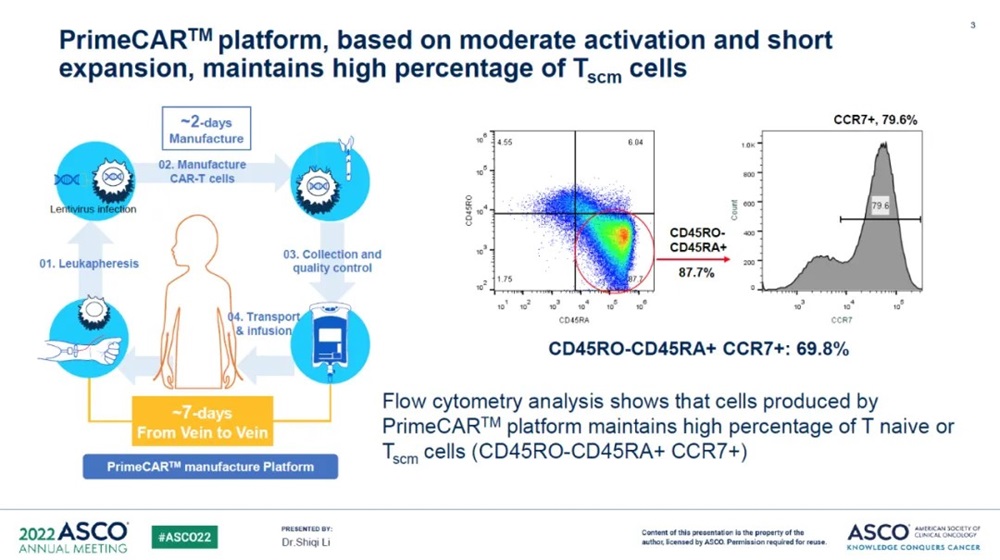
PRIMCAR platform of Chongqing Precision Biotech: In order to solve the industry pain points and common problems of CAR-T side reaction and high preparation cost, Chongqing Precision Biotech developed a new generation of cell preparation technology. This technology platform can produce CAR-T cells within 48 hours. By cooperating with strict quality control system, patients can complete cell infusion within 7 days, which can reduce patients' waiting time, greatly improve production efficiency and reduce production costs. The cells produced by this platform have a high proportion of T Native cells, which can exert therapeutic effect with a very small infusion dose and show good safety. These characteristics make it possible for the cells produced by PRIMCAR platform to be administered out-patient in the future, and further reduce the hospitalization and side effect treatment costs of patients.
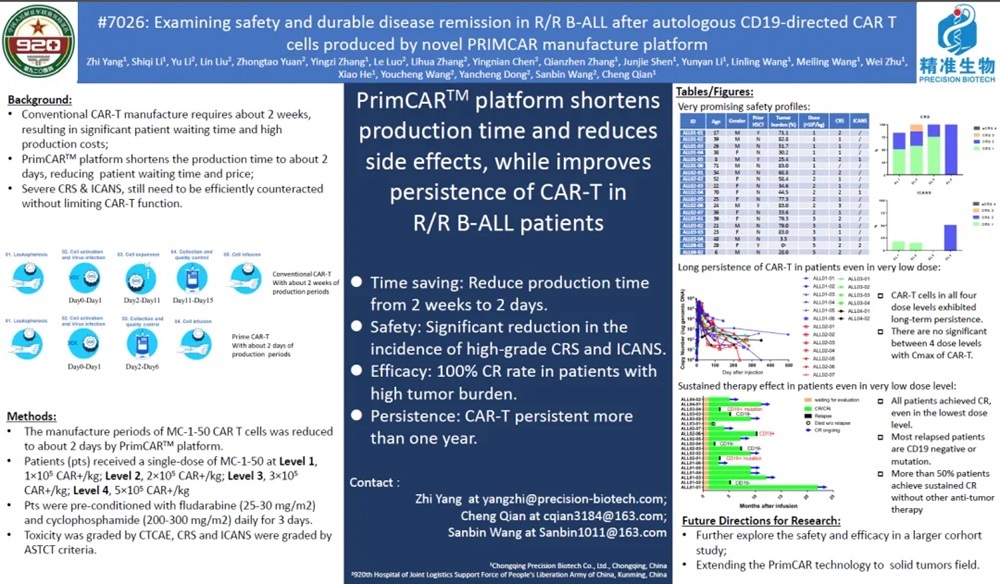
Clinical research data of MC-1-50: The clinical research data of MC-1-50 product developed based on PRIMCAR platform was first published in oral report session of Clinical Science Symposium of ASCO Annual Meeting in 2022. This report summarizes the safety and effectiveness of 13 patients with r/r B-ALL treated with MC-1-50. The average myeloma burden of the enrolled patients reached 58.4%, and the results showed that the ORR reached 100%. Eleven pts (84%) experienced CRS, including 7 (54%) at grade 1 and 4 (30%) at grade 2, no ≥ 3 CRS were observed. One pts (7%) experienced grade 1 ICANS, no ≥ 2 ICANS occurred. No DLTs were reported. (Resource from Autologous CD19-directed CAR T cells produced by novel PrimCAR manufacture platform exhibit safety, efficacy, and long persistence profiles in relapsed/refractory B-lineage acute leukemia (r/r B-ALL)).
At the ASCO annual meeting in 2023, the company updated the clinical data of this product in r/r B-ALL indications. A total of 19 cases of r/r B-ALL were treated with MC-1-50. More than half of the patients had myeloma burden ≥50%(11/19), and the cell preparation time of all subjects was about 2 days.
18 pts (94.7%) experienced CRS, including 10 (52.6%) at grade 1, 7 (36.8%) at grade 2, 1 (5.3%) at grade 3, and no≥4 CRS were observed. 3 pts (15.8%) experienced ICANS, including 2 (10.5%) at grade 1, 1(5.3%) at grade 2, and no≥3 ICANS occurred.No DLTs were reported. ORR reached 100%, and more than 50% patients achieved continuous CR remission without transplantation. The main causes of recurrent patients are CD19 negative recurrence or CD19 mutation. Most diseases of r/r B-ALL patients progress rapidly and have a heavy tumor load. Traditional CAR-T therapy usually has great side effects. The MC-1-50 product prepared by PRIMECAR platform not only has a short preparation time, but also is obviously superior to the traditional CAR-T product with the same target in terms of safety, and most patients can still achieve long-term sustained remission without bridging transplantation, which brings new dawn to r/r B-ALL patients.
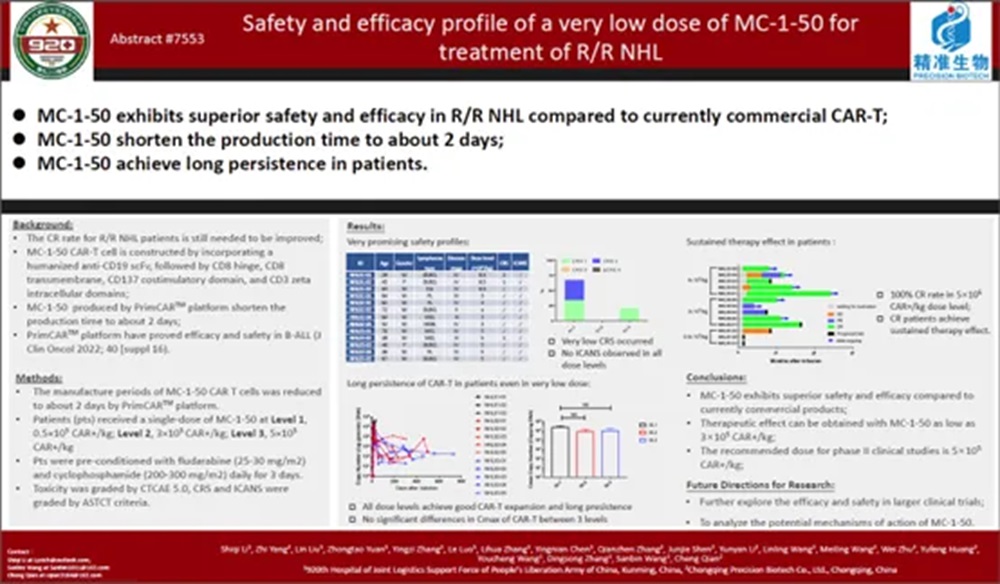
In addition, at the 2023 ASCO Annual Meeting, the Phase I clinical research data of MC-1-50 for the treatment of r/r B-NHL were also disclosed. A total of 13 cases of r/r B-NHL were included in the study, including DLBCL, CLL, FL, MCL, and HGBL. The cell preparation time for all subjects was about 2 days. Only 23% of patients experienced CRS, of which 2 patients (15%) experienced grade 1 CRS, 1 patient (8%) at grade 2, and no CRS≥3 was observed. ICANS was not observed in all patients. No DLTs were reported. All patients in the 5*10^5 CAR+/kg dose level achieved CR, and the subjects who achieved CR were able to achieve longer-lasting sustained remission, suggesting that patients in this dose group benefited better.
In terms of both therapeutic efficacy and safety, the treatment data of MC-1-50 in r/r B-ALL and r/r B-NHL patients are significantly better than other publicly available CAR-T products with the same target. At the same time, it also officially brings the time for CAR-T preparation Vein-To-Vein to less than one week, providing a safer, more timely and effective new option for patient treatment.