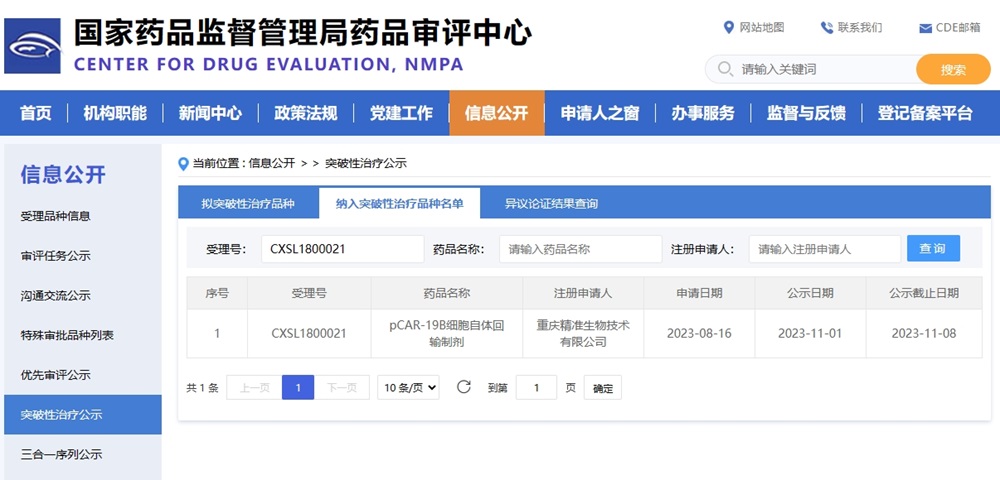
On November 10th,Precision Biotech today announced that the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) has granted Breakthrough Therapy Designation (BTD) for pCAR-19B, an CAR-T cell autologous infusion preparation, for treatment of CD19-positive relapsed/refractory acute lymphoblastic leukemia (B-ALL) aged from 3 to 21 years. This product is expected to be available in 2024.
About the Breakthrough Therapy Designation
In order to encourage the research and creation of drugs with obvious clinical advantages, National Medical Products Administration Drug Evaluation Center (CDE) issued the Announcement of NMPA on Issuing the Announcement on Three Documents Including the Working Procedures for Review of Breakthrough Therapy Drugs (Interim) (No.82, 2020).
In this document, the scope of breakthrough therapy is defined as: innovative drugs or improved new drugs that are used to prevent and treat diseases that seriously endanger life or seriously affect the quality of life during clinical trials, and there is no effective prevention method or sufficient evidence to show obvious clinical advantages compared with existing treatment methods. CDE will give priority to the communication and exchange of drugs included in Breakthrough Therapy Designation, provide guidance to sponsors and promote drug research and development.
Breakthrough of pCAR-19B
At present, there is no CAR-T product approved for the treatment of relapsed/refractory B-cell acute lymphoblastic leukemia in children and adolescents in China. pCAR-19B, as the first CAR-T product with this indication entering the critical clinical research stage in China, was included in Breakthrough Therapy Designation this time. Based on the outstanding clinical efficacy and patient benefits of its registered clinical research, compared with the existing treatment methods, it significantly improves the objective remission rate.
At present, the key clinical research of pCAR-19B has reached the main end point. We look forward to the publication of more meaningful relevant results of pCAR-19B, which will be available as soon as possible, so as to provide an effective new choice for children and adolescents with relapsed/refractory B-cell acute lymphoblastic leukemia.
About pCAR-19B
pCAR-19B is independently developed by Chongqing Precision Biotech under invention patent protection. It is a cellular immunotherapy product developed for malignant hematological diseases originated from CD19 positive B cells, and it is also the first humanized CAR-T product designed for this indication in China. At the same time, a safer gene transduction vector system is adopted, which has better effectiveness and safety. In February, 2019, pCAR-19B obtained the implied license for clinical trials of a Class I CAR-T cell biological new drug from National Medical Products Administration.
In November 2019, its Phase I clinical research was officially launched in Tongji hospital, affiliated to Tongji Medical College of Huazhong University of Science and Technology, with Professor Zhou Jianfeng as PI (principal investigator). This registered trial is also the first CAR-T registered clinical trial for children and adolescents with B-cell acute lymphoblastic leukemia. The data of Phase I clinical trial showed that all 9 patients in the group achieved complete remission (CR), with an overall remission rate of 100%, and the patients who achieved complete remission (CR) for the first time also had negative minimal residual disease (MRD). No dose-limited toxicity (DLT) and treatment-related deaths occurred, and the overall safety and tolerance were good.
In February, 2022, Phase II registered clinical trials were officially launched in Beijing Children's Hospital affiliated to Capital Medical University and Tongji hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology. Professor Wang Tianyou and Professor Huang Liang were the leading PI, and clinical trials were successively conducted in hematology departments of more than 10 3A hospitals in China, aiming at better evaluating the effectiveness and safety of pCAR-19B.
Up to now, Precision Biotech has obtained implied licenses for four Class I CAR-T cell biological new drugs and seven indications and the registered clinical research is progressing smoothly and excellent clinical data have been obtained. pCAR-19B is not only aimed at patients with CD19-positive relapsed/refractory B-ALL aged from 3 to 21 , but also approved for clinical research in China for other indications, including: patients with CD19-positive relapsed/refractory B-cell acute lymphoblastic leukemia aged from 22 to 70; patients aged 75 and under with relapsed/refractory CD19 positive diffuse large B-cell lymphoma, follicular lymphoma and mantle cell lymphoma.
The successful approval of pCAR-19B is a new breakthrough for Precision Biotech and the beginning of a new era. It is the initial intention and mission of our all employees to speed up the research and development and transformation of gene and cell drug therapy for malignant tumors and benefit more patients!




