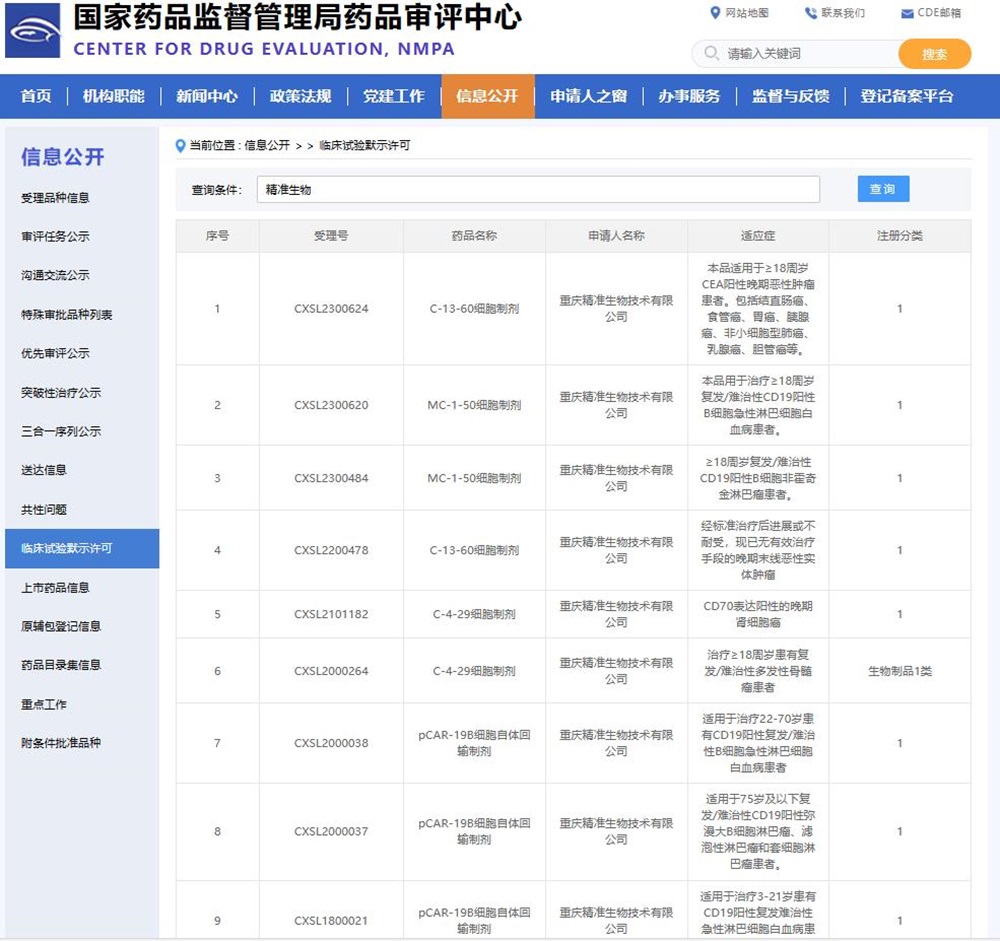
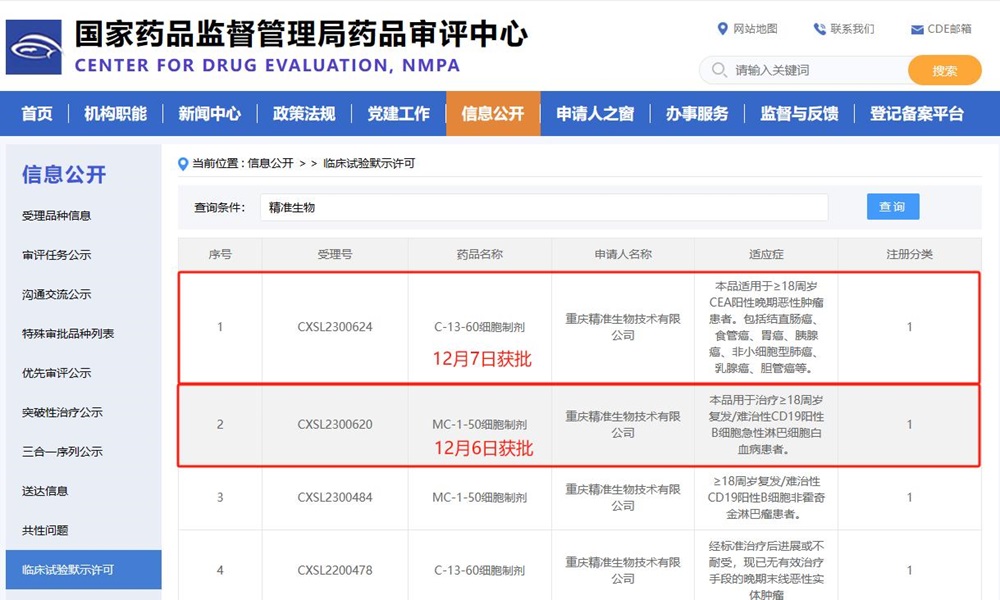
Following yesterday's approval of IND registration clinical license for MC-1-50, today, the application for clinical trial of C-13-60 cell preparation of Precision Biotech targeting CEA solid tumor was approved by National Medical Products Administration (NMPA). This new indication is intraperitoneal local medication, which is used for patients over 18 years old with CEA-positive advanced malignant tumors, including colorectal cancer, esophageal cancer, gastric cancer, pancreatic cancer, non-small cell lung cancer, breast cancer and cholangiocarcinoma. The first indication of C-13-60 cell preparation was approved as intravenous injection in September 2022, which was used for advanced or intolerant late-stage malignant solid tumors with no effective treatment.
Breaking through the limitations of local drug delivery CAR-T therapy
Yesterday, the second indication of MC-1-50, the first product of PRIMCAR® platform, which was independently developed by Precision Biotech to solve the problems of CAR-T side effects and high preparation cost, was granted the registered clinical implied license. Precision Biotech is not only deeply researched and developed in hematological tumors, but also continuously broken through in solid tumors. This clinical study is a local drug delivery which was different from the traditional drug delivery method.At present, the curative effect of CAR-T cell therapy in solid tumors is limited, which leads to its slow clinical application. Due to the inherent physical barrier of solid tumor, tumor immunosuppression microenvironment and other objective factors, CAR-T cells can not directly contact with solid tumor cells by conventional intravenous infusion, which leads to limited curative effect. Local administration can break through the limitations of traditional intravenous infusion, improve the problem of CAR-T transport and infiltration, effectively promote CAR-T cells to produce systemic immune function and better enter the tumor site, and establish a circulating and lasting immunity by inducing the tumor immune microenvironment to change into a microenvironment suitable for systemic immunity. At the same time, it can promote the migration and homing of CAR-T cells to other metastatic sites, enhance their killing effect on solid tumors and prevent recurrence. Some research studies have confirmed that local administration may reduce the risk caused by off-target effect, and its advantages have been reflected in many in vitro and vivo experiments and clinical studies.

About CEA (carcinoembryonic antigen)
CEA(carcinoembryonic antigen) is a glycoprotein, which is usually expressed during the development of cancer embryos. It is overexpressed on the surface of many cancer cells, including colorectal cancer, non-small cell lung cancer and breast cancer. Except for low expression in intestinal epithelial cells, CEA is hardly expressed in other normal cells, and it is a high-quality target of solid tumors.
About C-13-60
C-13-60 candidate product comes from RESCAR and PHICAR platforms independently developed by Precision Biotech. These platform technology can promote the recruitment of CAR cells to tumor sites, overcome tumor inhibition immune microenvironment, improve the curative effect, and enhanced CAR-T cells’ survival in tumor environment. The company has applied for a number of invention patents. The study of C-13-60 in preclinical mice shows remarkable effectiveness, which is expected to help more patients with rectal cancer, gastric cancer and esophageal cancer and also promote the clinical breakthrough of CAR-T cell therapy in the field of solid tumors.

About Chongqing Precision Biotech
Up to now, we has obtained implied licenses for four Class I CAR-T cell biological new drugs and nine indications. This year alone, three new indications have been approved. Our CAR-T candidate product for childhood leukemia has been approved as "Breakthrough Therapy Designation", and is expected to be listed in 2024. Breakthrough has been made in preclinical research of other R&D pipelines, and excellent results have been achieved.