The 2022 American Society of Clinical Oncology (ASCO) Annual Meeting will be held in Chicago from June 3 to June 7. This world's top clinical oncology conference includes Clinical Science Symposium, Oral Abstract Session, Poster Discussion Session and other sessions. In this year, 18 researches from China got the opportunity do oral presentation, including 3 Clinical Science Symposium and 15 Oral Abstract Session. The clinical research results of the innovative CAR-T therapy MC-1-50 developed by Chongqing Precision Biotech based on the unique PrimeCARTM platform were selected into the Clinical Science Symposium section.

(Source: ASCO)
Since FDA firstly approved CAR-T therapy in 2017, 8 CAR-T drugs have been approved for the market worldwide. This type of cell therapy has brought a huge breakthrough in the treatment of malignant hematological tumors. Among them, CD19-targeted CAR-T therapy shows a good curative effect on r/r B-ALL. However, life-threatening side effects and high treatment costs limit the widespread availability of such breakthrough therapies.
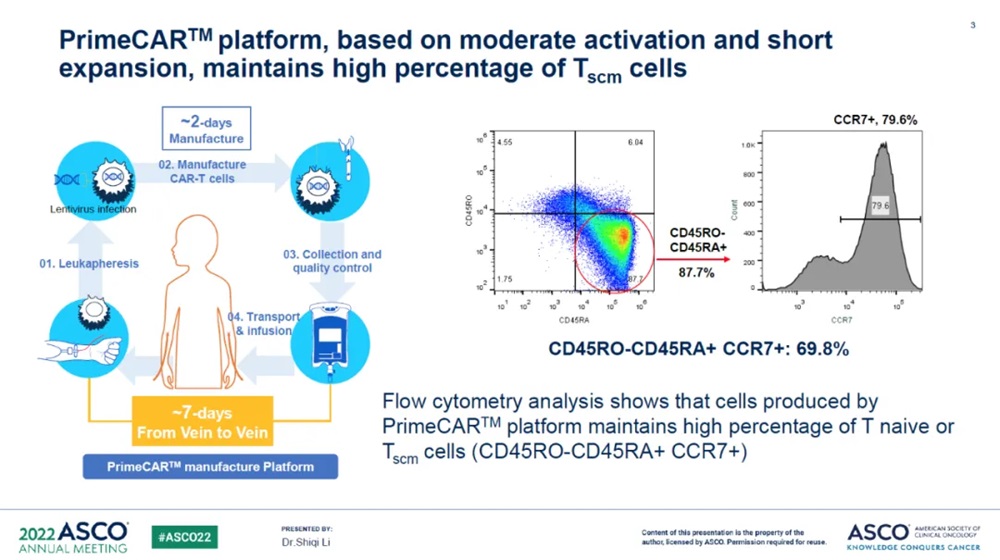
Manufacturing and Clinical Application Process of PRIMECAR® Platform and Phenotypic Assay of MC-1-50 (Source: ASCO)
Chongqing Precision Biotech's PrimeCARTM platform is a new generation of cell preparation process developed to address the two major pain points of CAR-T side reactions and high production costs. This platform can generate CAR-T cells within 48 hours and cooperate with a strict quality control system, so that patients can complete cell infusion within 7 days,which shorten the waiting time and greatly improved the production efficiency while reducing the production cost . In addition, the cells produced using this platform have a high proportion of TNative cells, allowing therapeutic effects at very small infusion doses to improve safety.
MC-1-50 was a CAR-T cell product targeting CD19 which developed on the PrimeCARTM platform. Preclinical studies have shown that this candidate product exhibits higher efficacy, lower toxic and side effects, and more durable in vivo protection efficacy than products produced with conventional preparations.
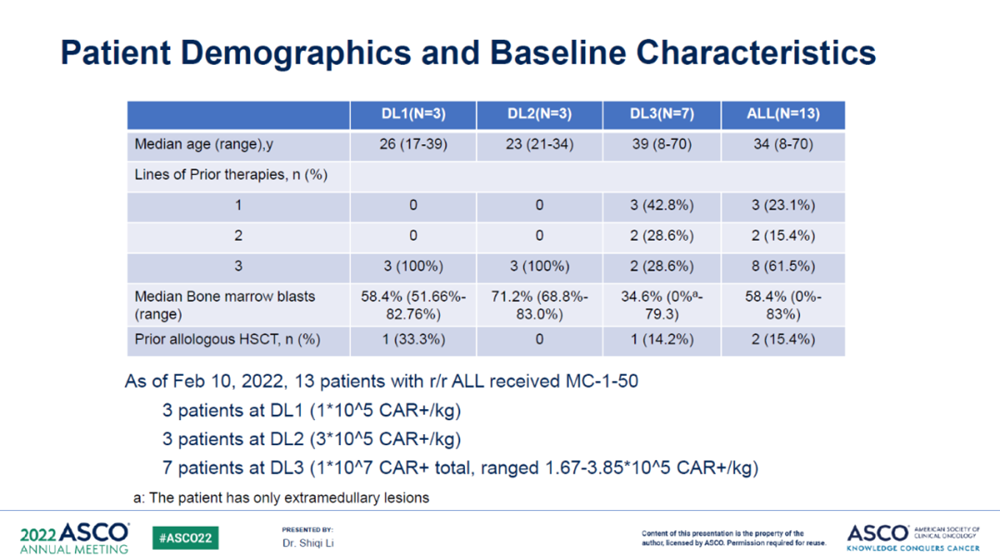
At this ASCO annual meeting, Chongqing Precision Biotech released the early results of a Phase I/II study (NCT04271410) on r/r B-ALL conducted in collaboration with the 920 Hospital of the Joint Logistics Support Unit of the People's Liberation Army. Patients (pts) received single-dose of MC-1-50 at dose level 1 (1×105 CAR+/kg), level 2 (3×105 CAR+/kg), and level 3 (1×107 CAR+ totally, ranged 1.67-3.85×105 CAR+/kg). Pts were pre-conditioned with fludarabine (25-30 mg/m2) and cyclophosphamide (200-300 mg/m2) daily for 3 days. Toxicity was graded by CTCAE, CRS and ICANS were graded by ASTCT criteria.
(Source: ASCO)
As of Feb 10, 2022, 13 pts with r/r B-ALL were infused with MC-1-50. The average myeloma burden of enrolled patients reached 58.4%, and no dose-limiting toxicities were found during treatment. Eleven pts (84%) experienced CRS, including 7 (54%) at grade 1 and 4 (30%) at grade 2, no ≥ 3 CRS were observed. One pts (7%) experienced grade 1 ICANS, no ≥ 2 ICANS occurred.
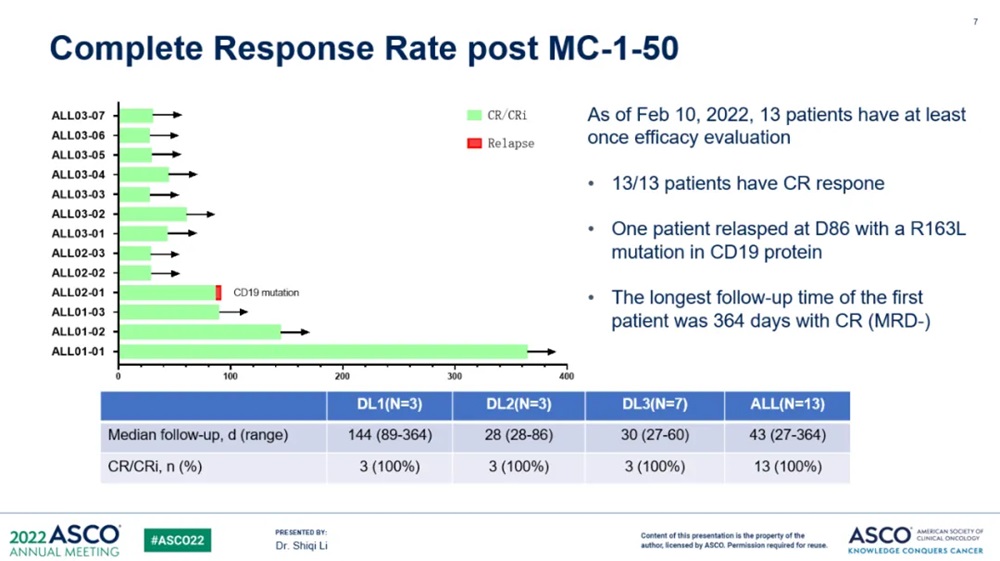
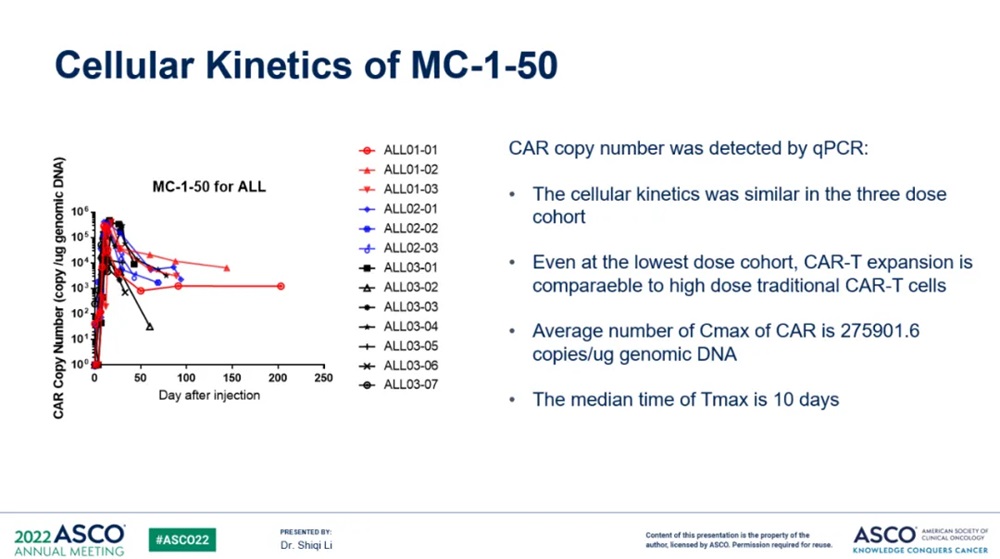
(Source: ASCO)
All 13 pts finished 1M evaluation and the CR rate in 1M is 100%. Four pts finished 3M evaluation and the CR rate in 3M is 75%, 1 pts relapsed at the month 3 with CD19 mutation. One pts had a CR statue at the month 11.
(Source: ASCO)
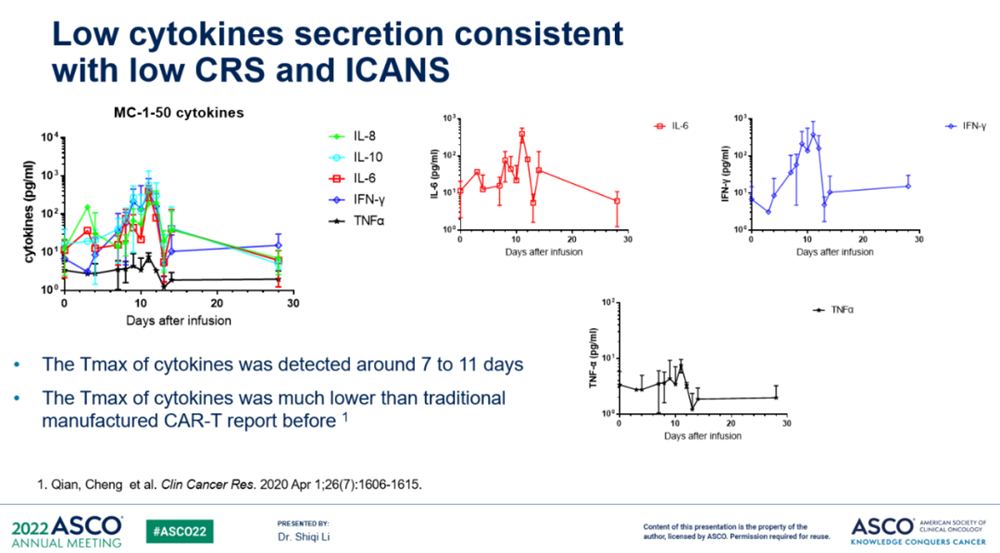
The tumor burden of the patients treated this time is relatively high, but in the case of high tumor burden, the incidence and grade of CRS and ICANS in patients are lighter than the side effects of CAR-T published in the past. By analyzing the level of cytokines in patients' peripheral blood, it was found that the level of cytokines induced by MC-1-50 produced by PrimeCARTM platform in the process of anti-tumor was significantly lower than that of traditional CAR-T products.
(Source: ASCO)
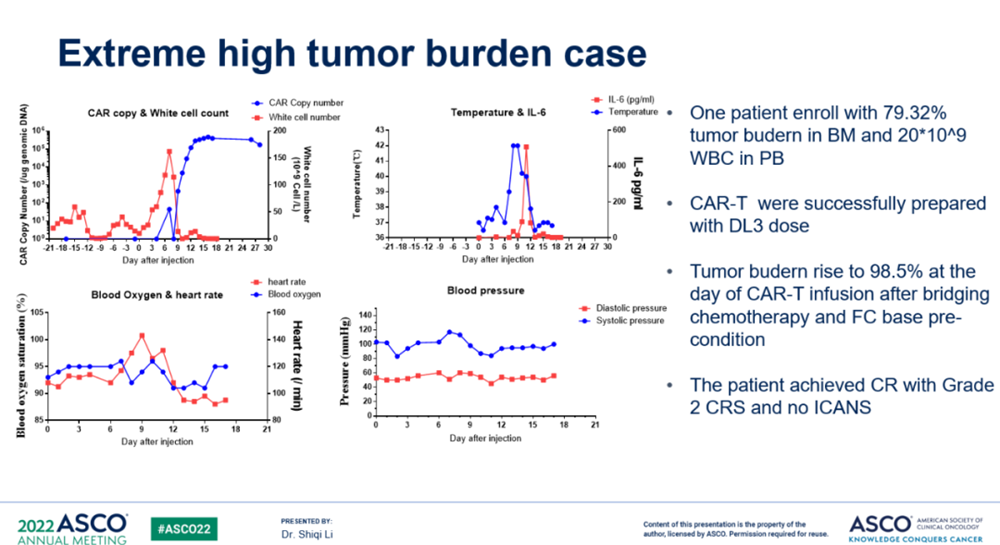
It is worth mentioning that among the 13 patients, there was 1 patient with a very high tumor burden, with a myeloma burden as high as 79.3% when entering the group, and peripheral blood was hyperwhite (the number of white blood cells reached nearly 50*109/L). The patient's CAR-T cells were successfully produced in the condition of hyperwhite peripheral blood. Bridging therapy was performed before the lymphoidectomy, and the tumor burden was briefly controlled. However, after the lymphoidectomy pretreatment, the tumor burden in the patient's bone marrow further increased to 98.5%, and the peripheral blood was still in a hyperleukosis state. After cell infusion, our patient had a rapidly increasing peripheral tumor burden with a peripheral white blood cell count up to 160 * 109/L. Seven days after CAR-T cell infusion, the peripheral blood tumor burden began to decrease, and the peripheral blood tumor was completely removed in about 12 days. The patient's bone marrow achieved complete remission after 34 days of re-transfusion. In terms of safety, with such a high tumor burden, our patient had only Grade 2 CRS and no ICANS.
Based on the above data, Professor Jeremy Mark Pantin, a famous hematology expert of the NIH in the United States, commented: "PrimeCARTM platform has the potential to shorten the patient's waiting time for transfusion, achieve a lower dose of transfusion, offer longer duration of CAR-T with a higher response rate and less toxic and side effects, and is expected to significantly reduce the recurrence rate of patients. It is a product worthy of expectation in the future." Professor Qian Cheng, chief scientist of Chongqing Precision Biotech, said: "Overall, our MC-1-50 product developed based on PrimeCARTM platform has shown the characteristics of high-efficiency production, low-dose infusion, and low toxic and side effects. While realizing the complete remission effect for patients with B-ALL with high tumor burden and refractory recurrence, it greatly controls the occurrence of CRS and ICANS, and offers the possibility for future outpatient treatment of CAR-T."
(Source: pharmcube)










