The C-13-60 cell preparation, a candidate product of Chongqing Precision Biotech, is a CEA CAR-T cell injection for the treatment of patients with CEA-positive advanced malignant solid tumors aged ≥18 years. Its clinical trial application has been submitted in September 2022. On March 22, it was accepted by NMPA.Its indications are for colorectal cancer, gastric cancer and esophageal cancer, and it is the first CEA-targeted CAR-T product accepted by CDE in China.

CEA (carcinoembryonic antigen) is a glycoprotein that is usually expressed during the development of cancer embryos and over-expressed on the surface of many cancer cells, including colorectal cancer, non-small cell lung cancer, and breast cancer. Except for the low expression in intestinal epithelial cells, CEA is almost not expressed in other normal cells and is an excellent target for solid tumors.

The C-13-60 candidate is derived from the RESCAR and PHICAR platforms independently developed by Chongqing Precision. These platforms can recruit CAR-T cells to the tumor site, overcome the tumor suppressive immune microenvironment and enhance the efficacy, while enhancing CAR-T cell survival in the tumor environment. The company has applied for a number of invention patents. The study of C-13-60 in preclinical mice shows remarkable effectiveness, which is expected to help more patients with rectal cancer, gastric cancer and esophageal cancer , and also promote the clinical breakthrough of CAR-T cell therapy in solid tumors.
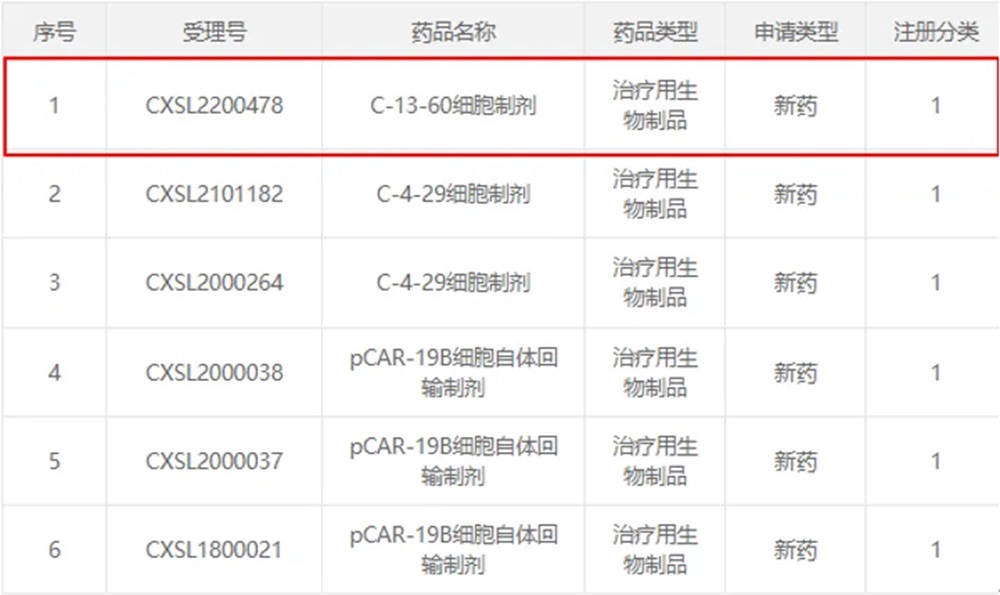
This clinical trial application is another CAR-T new drug clinical trial application for solid tumors following the clinical indication of C-4-29 cell preparation in 2021.Up to now, Chongqing Precision has applied 6 clinical trials and been accepted by CDE and obtained 5 clinical implied licenses. Among them, the product for children with acute lymphoblastic leukemia has entered clinical Phase II and has achieved excellent curative effect.






